Understand the biology of joint injuries that drives PTOA
When a joint is injured, mitochondria inside cartilage cells (chondrocytes) release excess reactive oxidant species (ROS). This oxidative stress damages and kills cells, triggering a chain reaction that degrades cartilage over time. CG-001 is being investigated for its potential to interrupt this process and preserve joint health.
How CG-001 is designed to work
Amobarbital, CG-001’s active ingredient, is a reversible inhibitor of Complex I in the mitochondrial electron transport chain. By reducing ROS production immediately after injury, it may help protect chondrocytes and maintain cartilage structure. This mechanism has been replicated in multiple large-animal studies, showing sustained joint protection.
Evidence from large animal studies
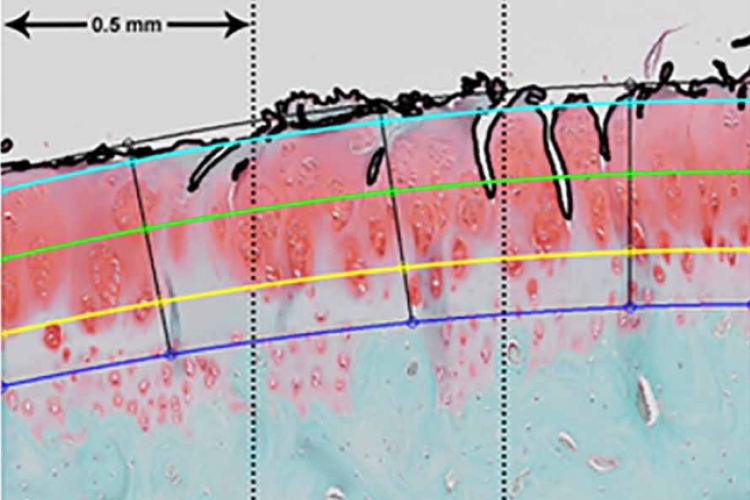
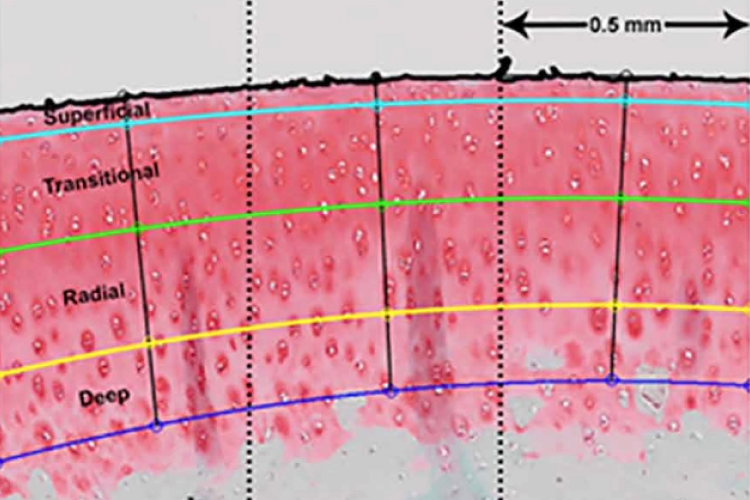
In a well-validated pig model of ankle fracture, researchers recorded the following findings after treatment with CG-001:
Fracture, No Treatment
Results after 6 months
Fracture + Amobarbital
Results after 6 months
6 months
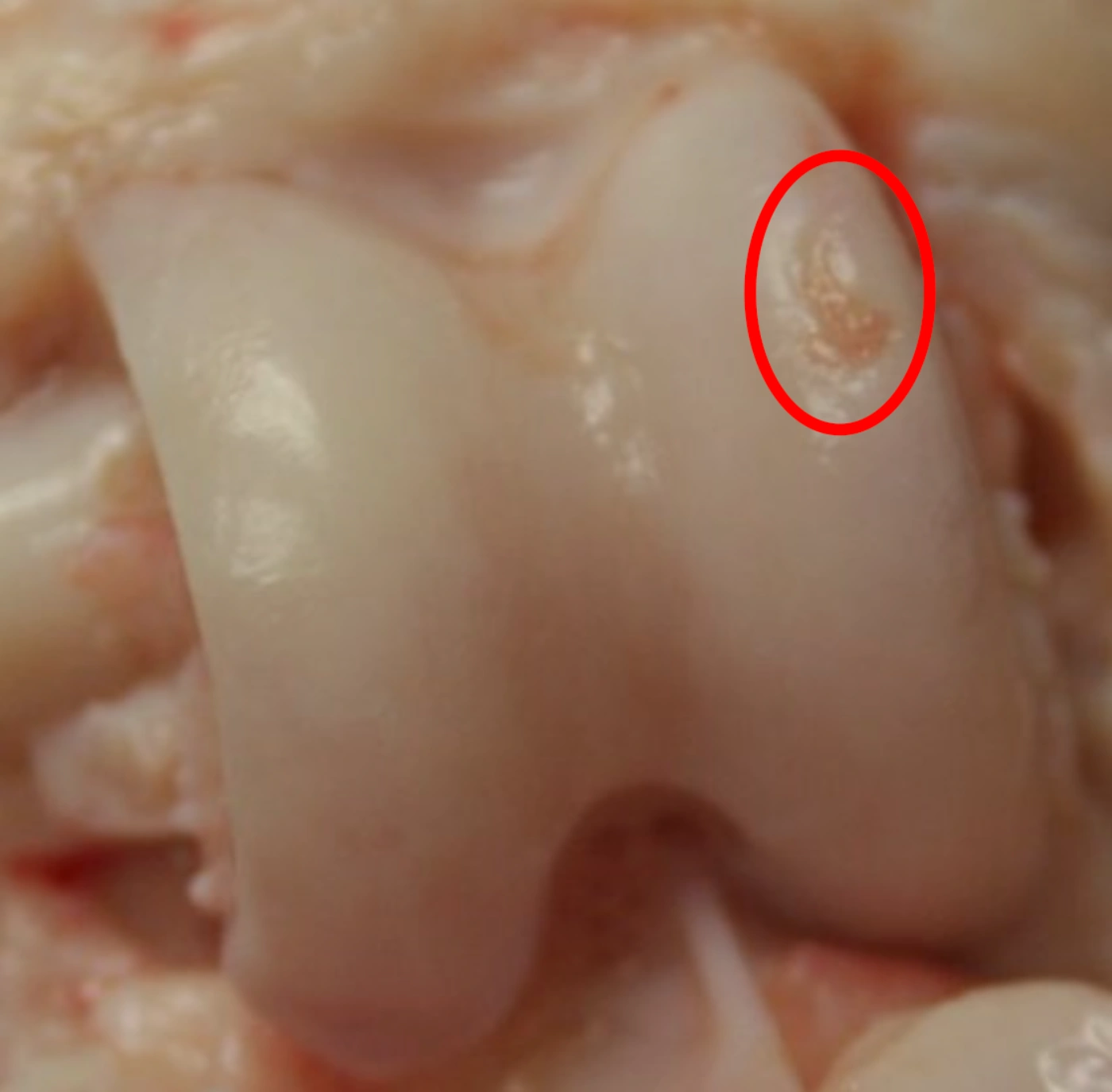
Histological analysis showed markedly healthier cartilage in joints treated with amobarbital.
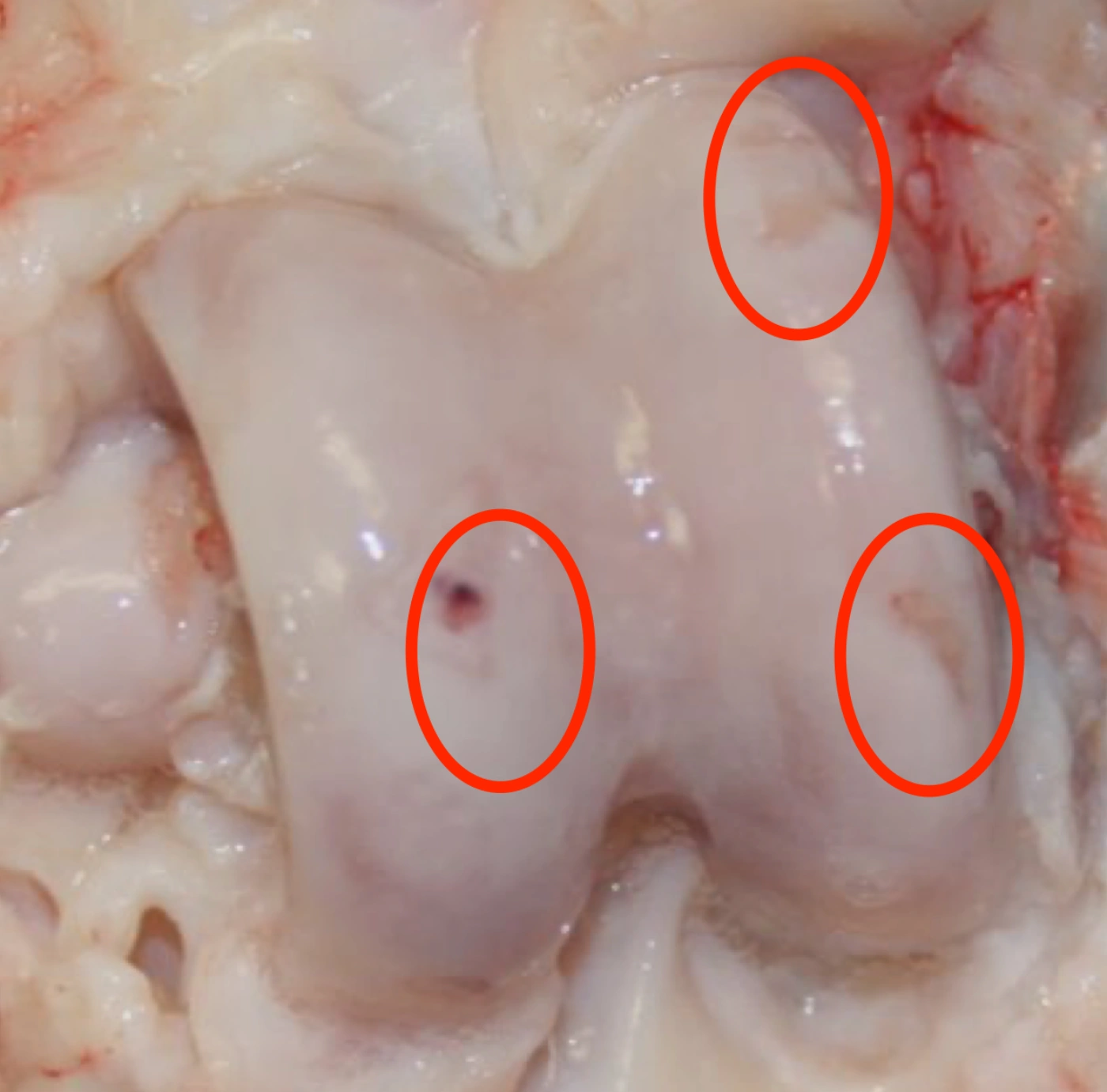
Fracture, No Treatment
Fracture + Amobarbital
12 months
Amobarbital treatment resulted in significantly fewer full-thickness cartilage lesions than untreated controls.
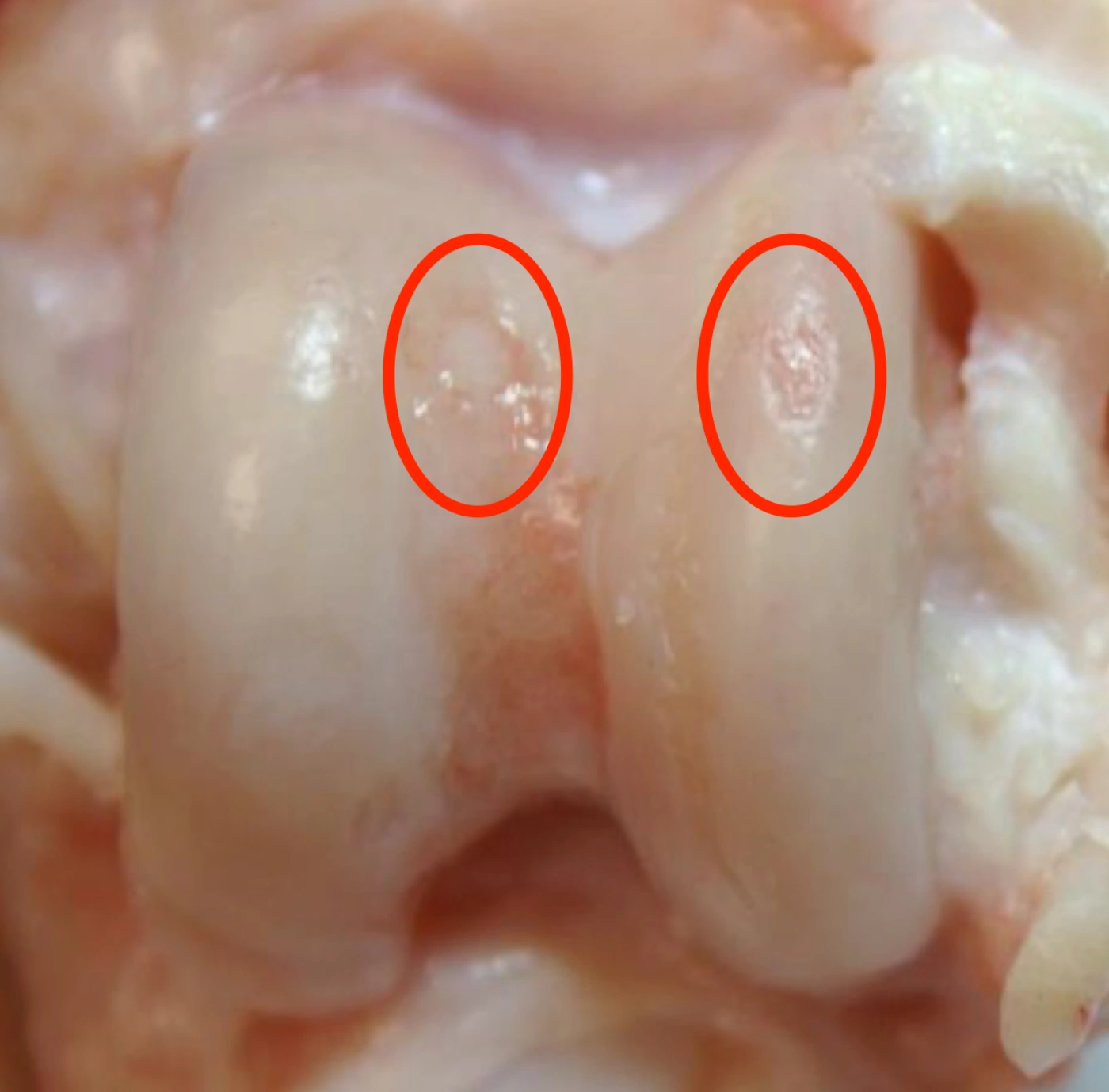
Fracture, No Treatment
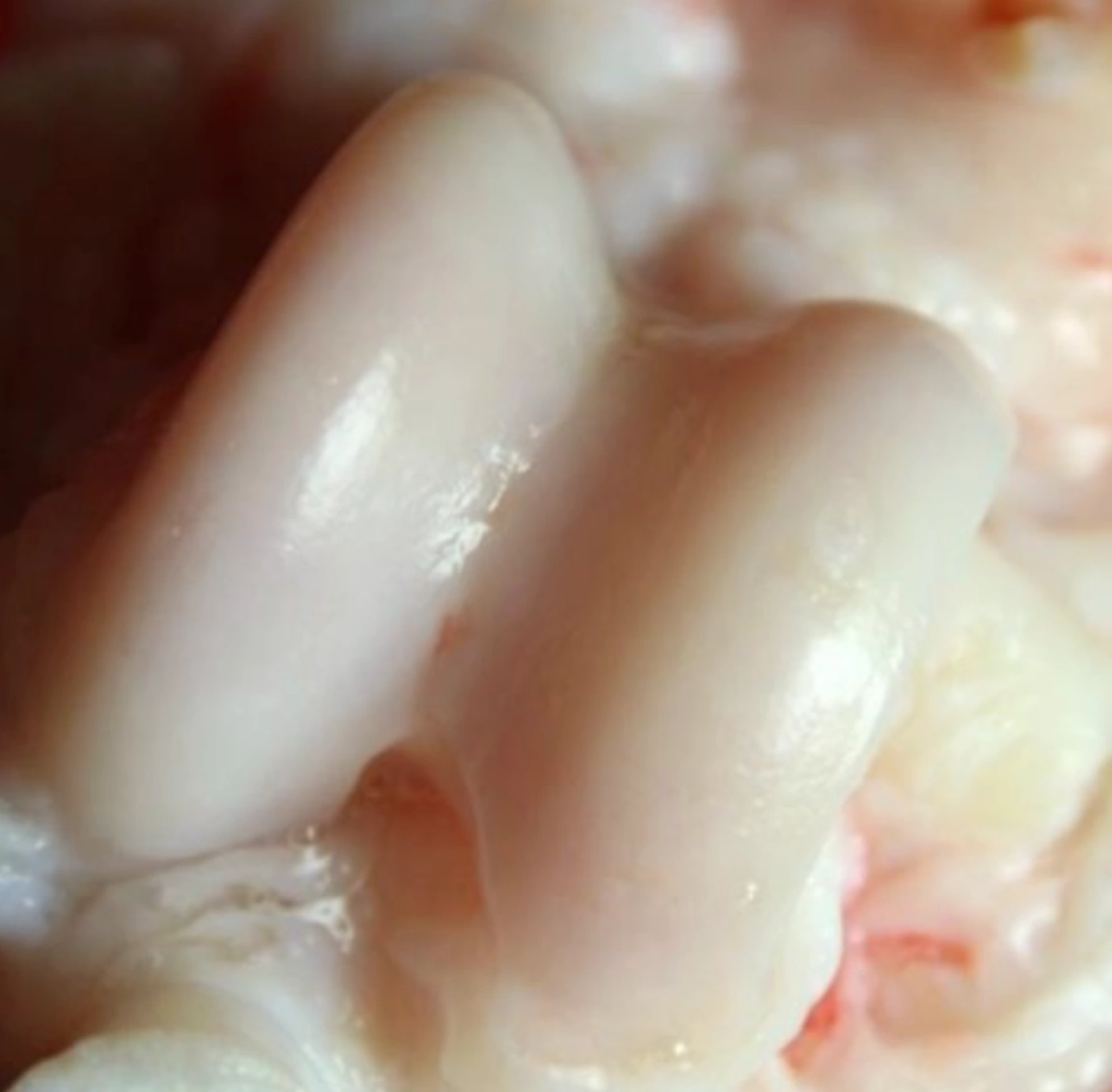
Fracture + Amobarbital
These results provide strong translational evidence for CG-001s protective effect on cartilage after joint injury.
Early clinical findings
Phase 1 testing in ankle fracture patients treated with CG-001 demonstrated:
- No adverse safety findings
- Normal biomarkers for organ function
- Confirmation of the safety profile seen in preclinical studies
These results provide a foundation to progress to a Phase 2 study of efficacy trial at multiple Level 1 trauma centers with Department of Defense support.

Upcoming clinical trial design at a glance
Our planned Phase 2 trial will enroll high-risk ankle fracture patients at multiple Level 1 trauma centers with Department of Defense support. It will assess the following endpoints:
- Joint structure using advanced weightbearing CT
- Radiographic grading with Kellgren-Lawrence scores
- Physician- and patient-reported functional outcomes at 6, 12, and 24 months
Note: Details will be finalized following discussions with regulatory authorities and funding partners.

Our scientific and clinical advisory board

Past President of Orthopedic Research Society and American Board of Orthopedic Surgery

Past Director of Ignacio V. Ponseti Biochemistry and Cell Biology Laboratory

Past President of American Orthopedic Association and American Board of Orthopedic Surgery

Principal Investigator for our planned Phase 2 clinical trial
Take the next step toward understanding the data
If you’re interested in the research behind CG-001 or opportunities to collaborate, we’re ready to share more.
